Operating model and organisational design
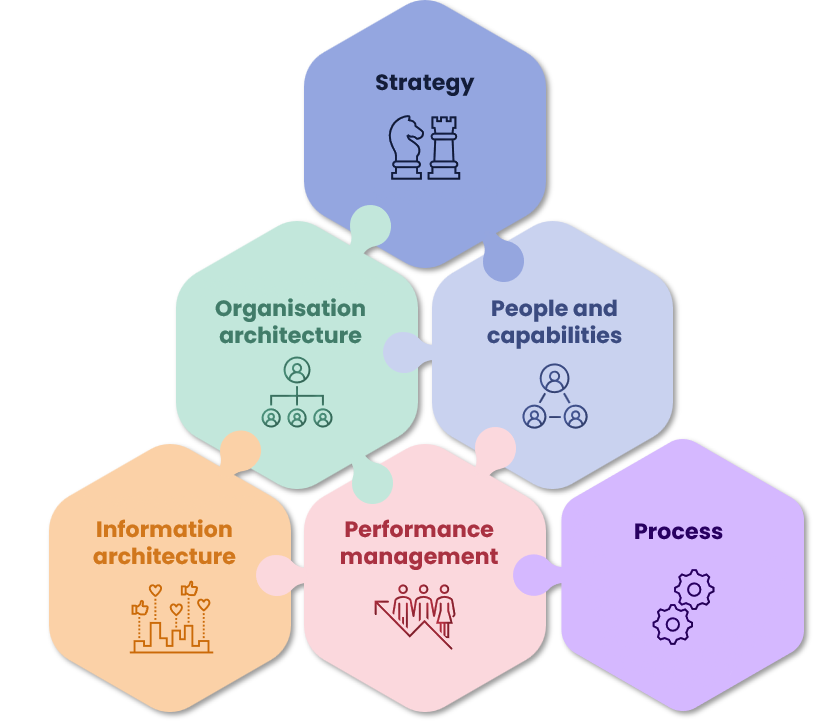
Protagoras works across the operating model to improve organisational effectiveness, providing:
- Strategy Development
- Operating Model Design and Implementation
- Project Management
- Change Management
Click on each of the areas below to read some examples of the work that we have done across each component of the operating model.
- Set and improved the portfolio strategy of a mid-sized pharma based on a data-driven approach, including investments, divestments, prioritisation and termination of development
- Due diligence of a strategic acquisition for a rapidly-growing biotech
- Support to strategy development of medical evidence generation team in a top-five pharma
- Mapping and redesign of an evidence generation planning approach and study design process, including relevant templates
- Benchmarking and redesign of ISS/CSS operation at a top-ten pharma
- Development and implementation of medical brand planning process, including supporting tools and templates
- Mapping and redesign of labelling process for a top-ten pharma
- Design of scientific communications outsourcing process for top-ten pharma
- Development of an overarching oncology Medical Affairs operating model and charter for top-ten pharma
- Operating model design with a clinical operations team, including core/non-core activity assessment
- Restructure of global Medical Affairs teams, including day-to-day operations and RACIs
- Project management support to the EMEA strategy and operations team of a mid-sized pharma, across ten workstreams
- Development and implementation of revised external engagement handbook and supporting training across 200+ field-based Medical Affairs team members
- Capability-mapping diagnostic for the medical oncology department of a mid-sized pharma
- Holistic systems and data architecture redesign at multiple top-10 pharma companies
- Design and implementation of clinical operations vendor management oversight and key performance indicators (KPIs)
- Design and implementation of clinical operations KPIs for vendor management
- Design and implementation of medical KPIs at the global, regional, and local levels
- Support to the overhaul of a mid-sized pharma’s governance and QMS landscape
- Gap analysis of a biotech QMS
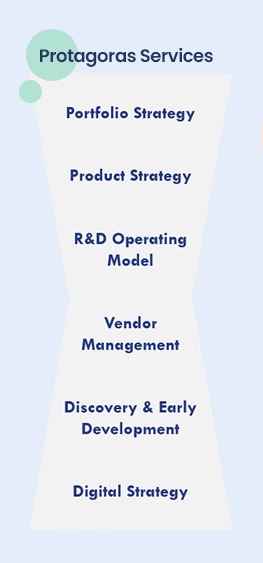
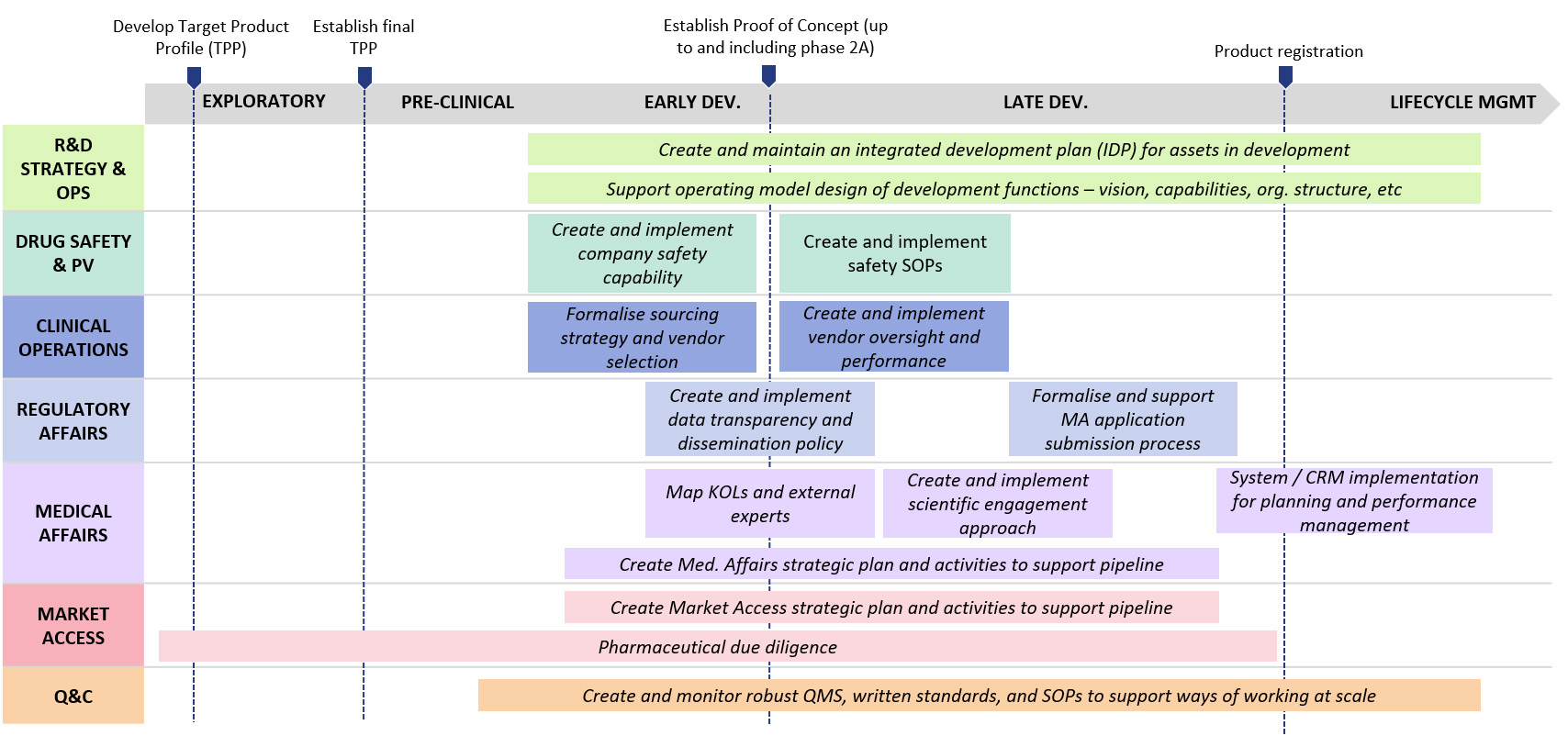
Protagoras’ expertise in clinical development and portfolio management supports top global pharma companies in building assets’ development strategy across the product lifecycle.
Protagoras has worked with companies to develop and optimise their portfolio strategy, including investments, divestments, prioritisation and termination of development
Protagoras has supported cross-functional product teams in building the clinical development strategy for assets in various indications and across the product lifecycle, to enable better decision-making earlier and mitigate risk
Protagoras has worked with R&D functions to institute vendor management best practices, vendor management dashboards, and vendor selection processes to ensure effective partnership whilst controlling cost
Protagoras teams have worked with research, early development, and translational science functions of top 20 pharma companies to optimise their operating models and the pre-clinical / clinical interface
Protagoras brings considerable experience in product & portfolio development strategy and understands the challenges that life science companies face both internally and externally.
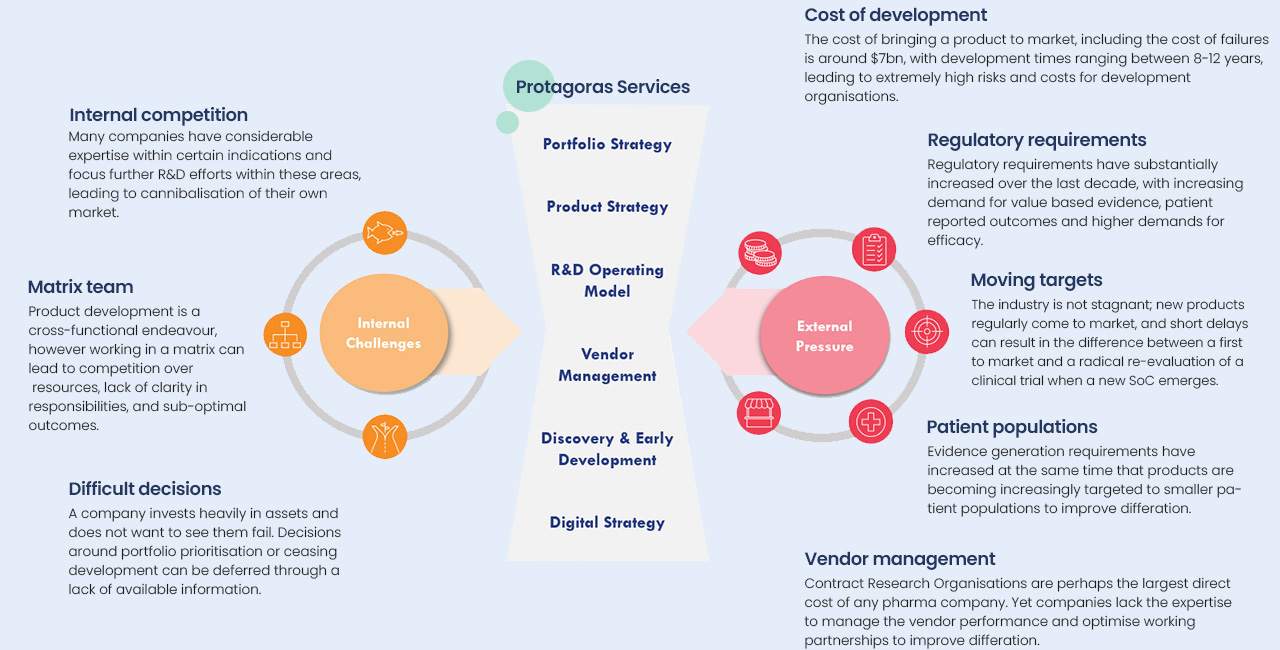
Many companies have considerable expertise with certain indications and focus further R&D efforts within these areas, leading to cannibalisation of their own market.
Product development is a cross-functional endeavour, however working in a matrix can lead to competition over resources, lack of clarity in responsibilities, and sub-optimal outcomes.
A company invests heavily in assets and does not want to see them fail. Decisions around portfolio prioritisation or ceasing development can be deferred through a lack of available information.
The cost of bringing a product to market, including the cost of failures is around $7bn, with development times ranging between 18-12 years, leading to extremely high risks and costs for development organisations.
Regulatory requirements have substantially increased over the last decade, with increasing demand for value based evidence, patient reported outcomes and higher demands for efficacy.
The industry is not stagnant; new products regularly come to market, and short delays can result in the difference between a first to market and a radical re-evaluation of a clinical trial when a new SoC emerges.
Evidence generation requirements have increased at the same time that products are becoming increasingly targeted to smaller patient populations to improve differation.
Contact Research Organisations are perhaps the largest direct cost of any pharma company. Yet companies lack the expertise to manage the vendor performance and optimise working partnerships to improve differation.
Biotech
Protagoras supports Biotech companies throughout the readiness roadmap.
Contact Us
- 020 8795 5047
- info@protagorasgroup.com
-
158-160 North Gower Street,
London NW1 2ND, United Kingdom
Send a message

